The Element M Forms the Chloride Mcl4
Which of the following statements about these chlorides is correct. Therefore 48 g is oxygen and 52 g is M.

Element M Forms A Chloride With Formula Mcl 4 Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve
558 u MCl4 M 4Cl-250g 750g Plan.

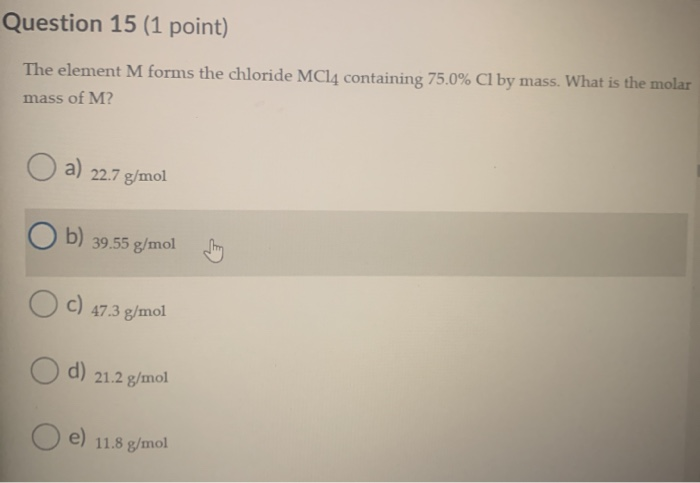
. 227 gmol 212 gmol 118 gmol 3955 gmol 473 gmol. What type of bond is in MCl 2. 1 Magnesium is used extensively in the form of alloys as a constructional material due to its low density 17gcm3 compared to 78gcm3 for iron.
A chemist has 250g sample of a transition metal and wishes to identify the metal. The element must have an oxidation of 2 which means group 2 elements. May 1 2021 thanh.
Up to 256 cash back A certain element X forms three different binary compounds with chlorine containing 5968 68 and 7475 chlorine respectively. The valency of chlorine is 1. GCl- molCl- molM AWM 75g Cl3545gmol-1 x 1molM4molCl- 0529 mol M mol gAW AWM gmol AWM 25 g0529 mol 473 gmol D.
A MCl 2 is more volatile than MCl 4 b MCl 2 is more soluble in anhydrous ethanol than MCl 4 c MCl 2 is more ionic than MCl 4 d MCl 2 is more easily hydrolysed than MCl 4. B is the only element among the given elements which has valency 3 and forms trigonal planar halide with chlorine. The bond angle in X is found to be 1 2 0 o.
Show how these data illustrate the law of multiple proportions. A metal M forms a compound with the formula MCl4. Element M is a metal that forms compounds of the type MX2 MX3 and MX4 where X is ChlorineCl.
If the compound is 6614 chlorine by weight what is the identity of M. 1 point for correct answer. The atomic radius of a barium atom is.
It is given that an element M reacts with chlorine to form a compound X. The element M forms the chloride text MCl_4. The element M forms the chloride MCl 4.
3955 gmol 118 gmol O 227 gmol O 473 gmol 212 gmol. The element M forms the chloride MCl4 containing 750 Cl by mass. The element must have an oxidation of 2 which means group 2 elements.
05273 dfrac2M_Cl2M_ClM 05273 dfrac235453235453M M. Element M forms a chloride with the formula MCl 2 which is solid with a high melting point. If the chloride contains 75 chlorine by mass calculate the molar mass of element M.
M 5584 g m o l. All of the lanthanide metals react with HCl to form compounds having the formula MCl2MCl3or MCl4 where M represents the metallic element. Advertisement Remove all ads.
She reacts the metal with excess HCl and obtains 427g of product. II Arrange these compounds in the order of decreasing ionic radius of M. Each Cl has a weight of 35 therefore four Cls has a combined weight of 140.
187 - 140 47 meaning the leftover weight of whatever X is 47. The element M forms the chloride MCl4 containing 54432 Cl by mass What is the identity of M. Write the formula of the compound when M combines with sulphur oxygen and nitrogen.
A Suggest the half-equation. This chloride contains 750 chloride by mass. It is usually prepared by the electrolysis of magnesium chloride MgC l2 at a temperature a little above its melting point of 715C.
The atomic radius of a barium atom is. Let us assume 100 g of the compound is present. Each metal forms a single compound.
Element M can be a choice of 4 elements Beryllium Magnesium Calcium or Strontium. Based on this information. An element M with a valence of 6 will form an oxide with the formula MO 3.
An unknown element reacts with chlorine to give the chloride MCl4. Hence M is a metal and belongs to group 2 of the periodic table. The element X forms the chloride XCl4 containing 750 Cl by mass.
2 970104MJ04 Answer all the questions in the spaces provided. What is the atomic mass of M. Therefore if the element M is forming a chloride of the formula MCl 2 it means that the valency of M is 2Also since the compound MCl 2 has high melting point it means that it is an ionic compound.
SHOW WORK 5 points. Element M can be a choice of 4 elements Beryllium Magnesium Calcium or Strontium. What is the molar mass of M.
So chlorine contain 54432 which is decod View the full answer Transcribed image text. 140 is 75 of 187 140075 187. Give the symbol So far I wrote out the balanced chemical equation as M 2Cl2 - MCl4 I see that the molar ratios between M and MCl4 are the same so I came up with 2596gMolar Mass MCl41248gMolar Mass M.
If the chloride contains 75 chlorine by mass calculate the molar mass of element M. An oxide of an element with a valance of 6 contains 48 oxygen. Chlorine has an atomic mass of 35453 amu.
Molar mass of the element the metal is lron Fe. I What is the expected trend in the ionic radius of M in these compounds. Solving the above equation we get the molar mass of the metal M 5584 g m o l e.
The element M forms the chloride MCl4 containing 54432 Cl by mass. Element M forms a Chloride with formula MCl4. What is the molar mass of M.
Express your answer as a chemical symbol. Element M would be most likely in the same group of periodic table as. What is the element X.
A Si B Al C Na D Mg. Molecular formula MCl4 let us assume that total mass of the the compound is 100. What is the atomic weight of this element.
If 1487 g of the unknown element gives 2596 g of MCl4 what is the element. The element M forms the chloride MCl 4 containing 750 Cl by mass. A metal M forms chlorides in 2 and 4 oxidation states.
Answer 1 of 11. The element M forms the chloride MCl4. This implies the compound is trigonal planar molecule of the type M C l 3.
Element M Forms A Chloride With The Formula Mcl2 Which Is Sold With High Melting Point To Which Group Of The Periodic Table Sarthaks Econnect Largest Online Education Community

Element M Forms A Chloride With Formula Mcl Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve

Solved The Element M Forms The Chloride Mcl4 Containing Chegg Com
Solved Question 15 1 Point The Element M Forms The Chegg Com

Solved The Element M Forms The Chloride Mcl4 Containing Chegg Com

Element M Forms A Chloride With Formula Mcl 4 Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve
A Metal M Forms Chlorides In 2 And 4 Oxidation States Which Of The Following Statements About These Chlorides Is Correct Sarthaks Econnect Largest Online Education Community
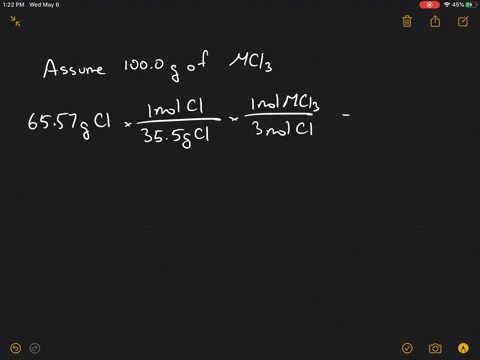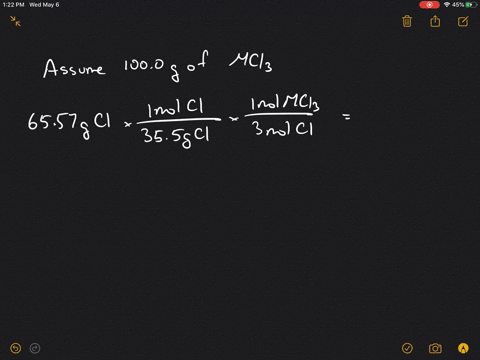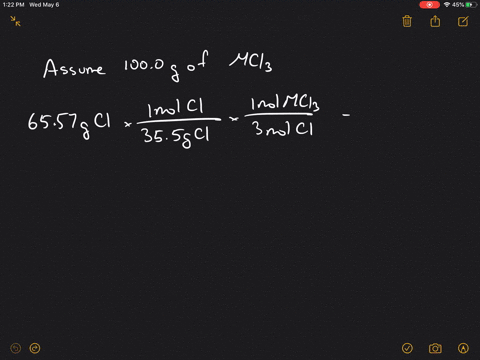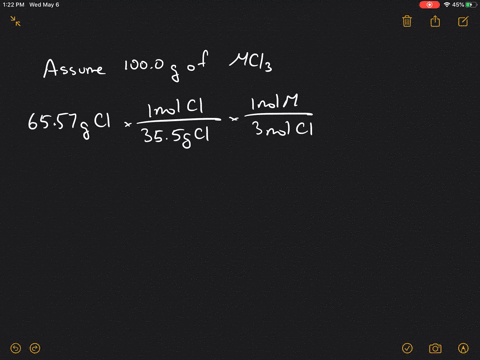
Solved When Aluminum Metal Is Heated With An Element From Group 6 Mathrm A Of The Periodic Table An Ionic Compound Forms When The Experiment Is Performed With An Unknown Group 6 Mathrm A Element

Element M Forms A Chloride With Formula Mcl Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve
Transition Metal Chloride Complex Wikipedia

Element M Forms A Chloride With Formula Mcl 4 Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve

Mc0 Mc1 Mc2 Mc3 Mc4 Mc5 Mc6 Mc7 0 If And Only If For Some Positive Integer K M
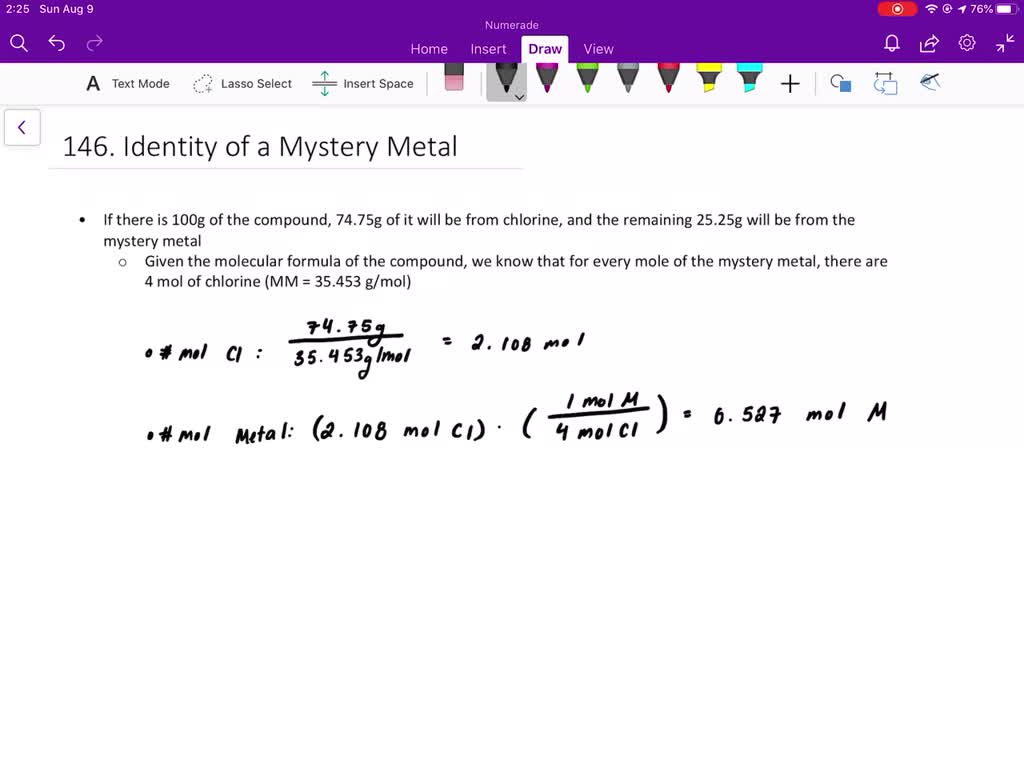
Solved 4 A Metal M Forms A Compound With A Formula Mcl3 If The Compound Contains 65 57 Cl Chlorine By Mass What Is Identity Of The Metal 5 A Chromium Containing Compound Has
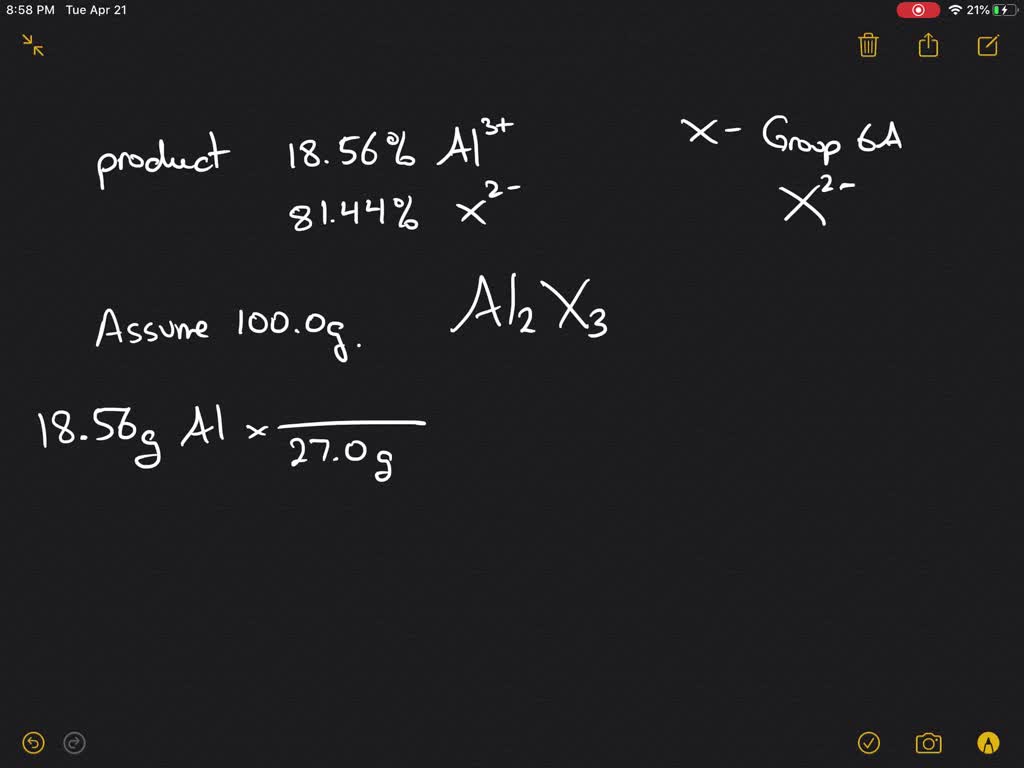
Solved When Aluminum Metal Is Heated With An Element From Group 6 Mathrm A Of The Periodic Table An Ionic Compound Forms When The Experiment Is Performed With An Unknown Group 6 Mathrm A Element

Element M Forms A Chloride With Formula Mcl 4 Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve

An Element M Reacts With Chlorine To Form The Compound Mci4 In This Compound No Lone Pairs Are Present And The Bond Angle Is 109 28 1 What Is M

Element M Forms A Chloride With The Formula Mcl Which Is A Scholr

Element M Forms A Chloride With Formula Mcl Element M Would Be Most Likely In The Samegroup Of Periodic Table As A Si B Al C Na D Mg Snapsolve

A Metal M Forms Chloride In Its 2 And 4 Oxidation States Which Of The Following Statements About These Chlorides Is Correct
Comments
Post a Comment